In Clarity AD, in which stage of AD were most of the patients?
Which stage of AD
The majority of patients were in the earliest symptomatic stage—MCI due to AD.1,2
For US Healthcare Professionals Only
An 18-month, global, placebo-controlled, double-blind, parallel-group, randomized clinical trial of 1795 patients with mild cognitive impairment (MCI) due to Alzheimer's disease (AD) or mild AD dementia with confirmed amyloid beta (Aβ) pathology. Patients were randomized 1:1 to receive infusions of LEQEMBI 10 mg/kg (n=898) or placebo (n=897) once every 2 weeks. The primary objective was to evaluate the effect of LEQEMBI as measured by CDR-SB.
A global, open-label, single-arm study evaluating LEQEMBI in patients with early AD for up to 48 months. Patients enrolled in the Clarity AD Core period (0-18 months) were given the option to participate in Clarity AD LTE (18-48 months); some remained on infusion while others were on different subcutaneous maintenance doses. The primary objective was to evaluate the long-term safety and whether the effect of LEQEMBI, as measured by CDR-SB at the end of the Clarity AD Core period, is maintained over time.
ADNI is a global (US and Canada) research study that follows the natural progression of AD, including imaging, biomarkers, genetics, and neurocognitive tests, and that actively evaluates the investigation and development of treatments for AD. ADNI is an untreated observational cohort (n=436 at baseline, followed for 48 months) that was a prespecified matched ADNI cohort used to design the Clarity AD study, based on demographics and clinical characteristics.4,5
In the Core placebo-controlled period (0-18 months), LEQEMBI achieved statistical significance, slowing progression by 27% vs placebo (P<0.0001).
Data at 4 years show continued separation in CDR-SB scores vs Alzheimer's Disease Neuroimaging Initiative (ADNI). About 94% of patients in the LEQEMBI arm who completed the Core period opted to continue in the LTE after 18 months.
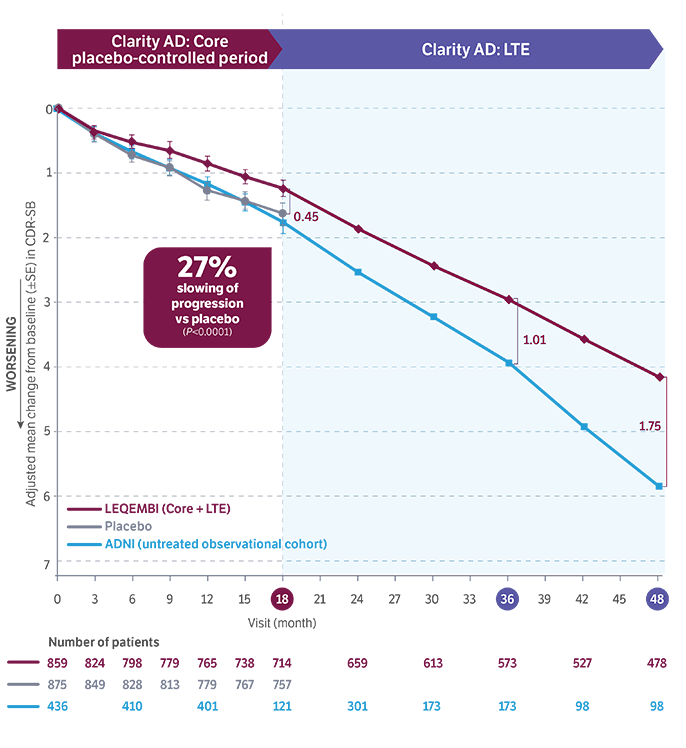
Cutoff: March 31, 2025.10
The LTE study is ongoing.11
ADNI data limitations: This retrospective analysis for ADNI should be interpreted carefully to determine a difference with LEQEMBI because of the potential selection bias and attrition.
AD, Alzheimer’s disease; ADNI, Alzheimer’s Disease Neuroimaging Initiative; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating-Sum of Boxes; LTE, long-term extension; PD, pharmacodynamics; PK, pharmacokinetics; SE, standard error.
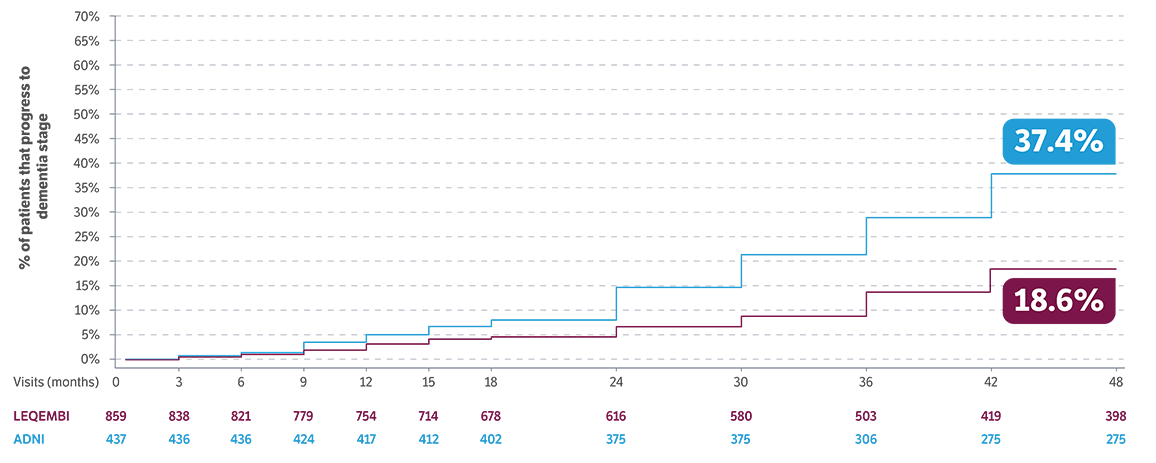

of patients remained in the MCI due to AD or mild AD stages through 4 years of treatment5,12
Moderate to severe AD dementia stages are associated with loss of independence and difficulty with daily activities13
Limitations: Patients were enrolled in the LTE after completion of the controlled period and are subject to continued dropout. After 18 months, data included patients on a range of subcutaneous doses, all within the FDA bioequivalence acceptance criteria (PK/PD).4,6
ADNI data limitations: This retrospective analysis for ADNI should be interpreted carefully to determine a difference with LEQEMBI because of the potential selection bias and attrition.
Early AD and low tau data
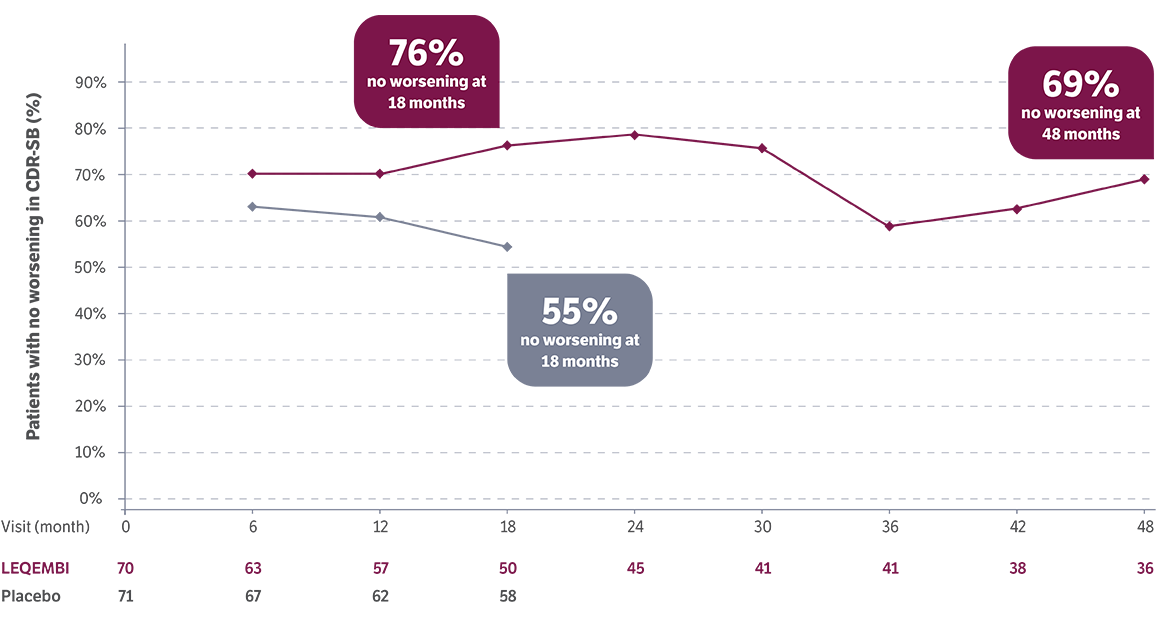
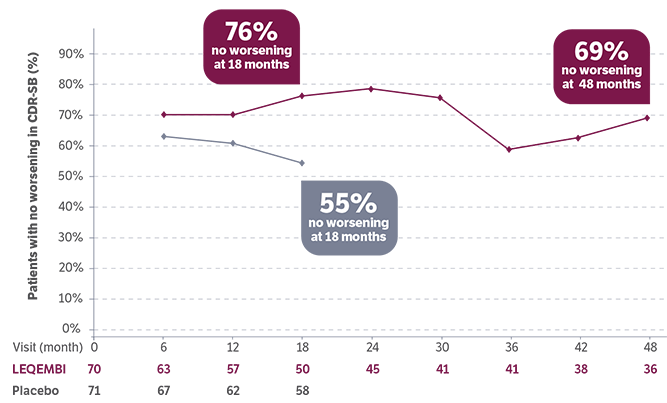
LEQEMBI patients with early AD and low tau showed improvement in CDR-SB scores vs baseline*5
At 18 months:
improved
(placebo 28%)
At 4 years:
improved
An increase in CDR-SB score=worsening and a decrease in score=improvement14
*No placebo post 18 months during the LTE.5
Limitations: These are post hoc exploratory analyses data from the tau PET subgroup of the Clarity AD study. These analyses were limited by small sample size and no conclusions can be drawn. After 18 months, data included patients on a range of subcutaneous doses, all within
the FDA bioequivalence acceptance criteria (PK/PD).6,14
Clarity AD: Post hoc analyses of CDR-SB scores in a low tau patient population14
Study description: The predefined optional tau PET substudy looked at outcomes of CDR-SB results across tau levels stratified by the participants’ level of the brain tau aggregates (tau PET), using the Cerveau database, as well as correlations of tau data to clinical outcomes (n=342).
Results of post hoc analysis:
Overall tau PET substudy (placebo, 167; LEQEMBI, 175): Adjusted mean difference vs placebo: -0.52 (95% CI: -1.01, -0.04)14,15
Low tau PET (<1.06): (placebo, 71; LEQEMBI, 70): Adjusted mean difference vs placebo: -0.59 (95% CI: -1.09, -0.09)14,15
Intermediate tau levels (n=191; SUVR ≥1.06-≤2.91) and high tau levels (n=10: SUVR >2.91): (placebo, 96; LEQEMBI, 105): Adjusted mean difference vs placebo: -0.49 (95% CI: -1.19, 0.21)14,15
As shown, further analyses were conducted in the low tau group to measure the change from baseline on the CDR-SB14

Lower levels of tau accumulation, as identified by SUVR, were found to be indicative of a patient with earlier stages of disease.13,14
No testing for tau is required to start LEQEMBI.1
CI, confidence interval; PET, positron emission tomography; SUVR, standardized uptake value ratio.
An overview of the steps involved from diagnosis through treatment with LEQEMBI (for eligible patients)
Biomarker endpoint: Plaque negativity
Key secondary endpoint: Change in Aβ PET Centiloid1
-59.1 Centiloid (CL) difference vs placebo at 18 months (P<0.00001)3
Prespecified biomarker endpoint percentage of patients achieving plaque negativity: Measured by conversion to PET amyloid negative (<30 CL)3
24%
OF PATIENTS
Placebo: 15%
LEQEMBI n=296
Placebo n=303
36%
OF PATIENTS
Placebo: 14%
LEQEMBI n=275
Placebo n=286
54%
OF PATIENTS
Placebo: 15%
LEQEMBI n=276
Placebo n=259
68%
OF PATIENTS
Placebo: 16%
LEQEMBI n=210*
Placebo n=205*
Limitations: Prespecified biomarker endpoint was not adjusted for multiplicity, therefore, no conclusions or comparisons can be drawn.
*73 subjects were not included at 18 months (per the statistical analysis plan) since their PET assessments were performed after receiving LEQEMBI in the extension phase.3

Continuous administration of LEQEMBI works in 2 ways to remove insoluble and soluble Aβ1,3
Which stage of AD
The majority of patients were in the earliest symptomatic stage—MCI due to AD.1,2
LTE data inclusive
Yes, patients receiving both IV and different subcutaneous maintenance doses were included in the LTE data.5
patient population
This cohort represents patients with early AD and low tau levels.13,14