SUMMARY OF IMPORTANT SAFETY INFORMATION
INDICATION
LEQEMBI® is indicated for the treatment of Alzheimer's disease. Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.
SUMMARY OF MOST SERIOUS AND MOST COMMON RISKS ASSOCIATED WITH LEQEMBI
BOXED WARNING: AMYLOID-RELATED IMAGING ABNORMALITIES (ARIA)
CONTRAINDICATION: Contraindicated in patients with serious hypersensitivity to lecanemab-irmb or to any of the excipients. Reactions have included angioedema and anaphylaxis.
WARNINGS AND PRECAUTIONS: ARIA; HYPERSENSITIVITY REACTIONS; INFUSION-RELATED REACTIONS
MOST COMMON ADVERSE REACTIONS: infusion-related reactions, ARIA-H, ARIA-E, headache, superficial siderosis of central nervous system, rash, and nausea/vomiting.
Injection-site reactions have occurred with LEQEMBI IQLIK.
Please see full Important Safety Information below and accompanying full US Prescribing Information, including Boxed WARNING.
Alzheimer's disease is a devastating chronic, progressive disease which results in the decline of both cognitive abilities and daily functioning.
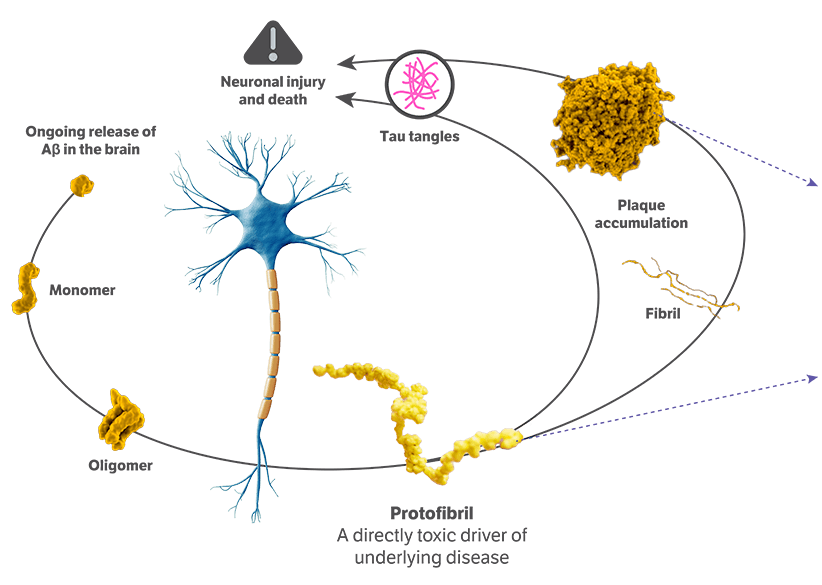
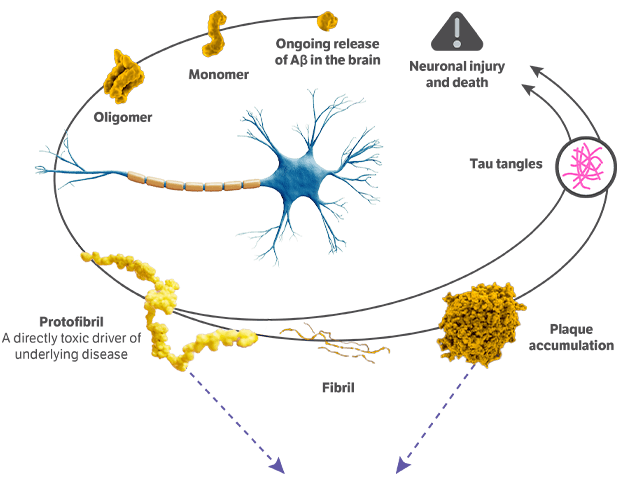
It is caused by the degeneration and death of brain cells critical for processing, storing, and retrieving information. One key hallmark of the disease is the abnormal accumulation of a protein called amyloid beta, which results from an imbalance of its production and clearance. Amyloid beta builds up in 2 forms, known as aggregated soluble and insoluble proteins.
Mild cognitive impairment is the first symptomatic stage of the disease where memory and cognitive functions begin to be impaired but not severe enough to significantly disrupt daily life.
LEQEMBI is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody and the first fully approved FDA treatment that works on fighting Alzheimer's disease in 2 ways. First, LEQEMBI targets and clears insoluble amyloid beta plaques that have been accumulating for years. Second, LEQEMBI works to clear toxic, soluble aggregates of amyloid beta proteins such as oligomers and protofibrils.
LEQEMBI is an early Alzheimer's disease treatment designed to selectively target toxic protofibrils in addition to amyloid plaques. As a result of LEQEMBI binding to these toxic forms of the amyloid beta, it enables the microglia, the brain's immune cells, to recognize the toxic proteins and clear them out of the body via phagocytosis.
Early detection of abnormal amyloid accumulation via biomarker analysis, in addition to clinical symptoms, is critical in the diagnosis of patients with mild cognitive impairment due to Alzheimer's disease who may be appropriate for LEQEMBI.
INDICATION
LEQEMBI® is indicated for the treatment of Alzheimer's disease (AD). Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment (MCI) or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.
IMPORTANT SAFETY INFORMATION
WARNING: AMYLOID-RELATED IMAGING ABNORMALITIES (ARIA)
Monoclonal antibodies directed against aggregated forms of beta amyloid, including LEQEMBI, can cause ARIA, characterized as ARIA with edema (ARIA-E) and ARIA with hemosiderin deposition (ARIA-H). Incidence and timing of ARIA vary among treatments. ARIA usually occurs early in treatment and is usually asymptomatic, although serious and life-threatening events, including seizure and status epilepticus, can occur. ARIA can be fatal. Serious intracerebral hemorrhages (ICH) greater than 1 cm, some of which have been fatal, have been observed with this class of medications. Because ARIA-E can cause focal neurologic deficits that can mimic an ischemic stroke, consider whether such symptoms could be due to ARIA-E before giving thrombolytic therapy to a patient being treated with LEQEMBI.
Apolipoprotein E ε4, (ApoE ε4) Homozygotes: Patients who are ApoE ε4 homozygotes (approximately 15% of patients with AD) treated with this class of medications have a higher incidence of ARIA, including symptomatic, serious, and severe radiographic ARIA, compared to heterozygotes and noncarriers. Testing for ApoE ε4 status should be performed prior to initiation of treatment to inform the risk of developing ARIA. Prior to testing, prescribers should discuss with patients the risk of ARIA across genotypes and the implications of genetic testing results. Prescribers should inform patients that if genotype testing is not performed, they can still be treated with LEQEMBI; however, it cannot be determined if they are ApoE ε4 homozygotes and at higher risk for ARIA.
Consider the benefit of LEQEMBI for the treatment of AD and the potential risk of serious ARIA events when deciding to initiate treatment with LEQEMBI.
CONTRAINDICATION
Contraindicated in patients with serious hypersensitivity to lecanemab-irmb or to any of the excipients. Reactions have included angioedema and anaphylaxis.
WARNINGS AND PRECAUTIONS
AMYLOID-RELATED IMAGING ABNORMALITIES
Medications in this class, including LEQEMBI, can cause ARIA-E, which can be observed on MRI as brain edema or sulcal effusions, and ARIA-H, which includes microhemorrhage and superficial siderosis. ARIA can occur spontaneously in patients with AD, particularly in patients with MRI findings suggestive of cerebral amyloid angiopathy (CAA), such as pretreatment microhemorrhage or superficial siderosis. ARIA-H generally occurs with ARIA-E. Reported ARIA symptoms may include headache, confusion, visual changes, dizziness, nausea, and gait difficulty. Focal neurologic deficits may also occur. Symptoms usually resolve over time.
Incidence of ARIA
Symptomatic ARIA occurred in 3% and serious ARIA symptoms in 0.7% with LEQEMBI. Clinical ARIA symptoms resolved in 79% of patients during the period of observation. ARIA, including asymptomatic radiographic events, was observed: LEQEMBI, 21%; placebo, 9%. ARIA-E was observed: LEQEMBI, 13%; placebo, 2%. ARIA-H was observed: LEQEMBI, 17%; placebo, 9%. No increase in isolated ARIA-H was observed for LEQEMBI vs placebo.
Incidence of ICH
ICH greater than 1 cm in diameter was reported in 0.7% with LEQEMBI vs 0.1% with placebo. Fatal events of ICH in patients taking LEQEMBI have been observed.
Risk Factors of ARIA and ICH
ApoE ε4 Carrier Status
Of the patients taking LEQEMBI, 16% were ApoE ε4 homozygotes, 53% were heterozygotes, and 31% were noncarriers. With LEQEMBI, ARIA was higher in ApoE ε4 homozygotes (LEQEMBI: 45%; placebo: 22%) than in heterozygotes (LEQEMBI: 19%; placebo: 9%) and noncarriers (LEQEMBI: 13%; placebo: 4%). Symptomatic ARIA-E occurred in 9% of ApoE ε4 homozygotes vs 2% of heterozygotes and 1% of noncarriers. Serious ARIA events occurred in 3% of ApoE ε4 homozygotes and in approximately 1% of heterozygotes and noncarriers. The recommendations on management of ARIA do not differ between ApoE ε4 carriers and noncarriers.
Radiographic Findings of CAA
Neuroimaging findings that may indicate CAA include evidence of prior ICH, cerebral microhemorrhage, and cortical superficial siderosis. CAA has an increased risk for ICH. The presence of an ApoE ε4 allele is also associated with CAA.
The baseline presence of at least 2 microhemorrhages or the presence of at least 1 area of superficial siderosis on MRI, which may be suggestive of CAA, have been identified as risk factors for ARIA. Patients were excluded from Clarity AD for the presence of greater than 4 microhemorrhages and additional findings suggestive of CAA (prior cerebral hemorrhage greater than 1 cm in greatest diameter, superficial siderosis, vasogenic edema) or other lesions (aneurysm, vascular malformation) that could potentially increase the risk of ICH.
Concomitant Antithrombotic or Thrombolytic Medication
In Clarity AD, baseline use of antithrombotic medication (aspirin, other antiplatelets, or anticoagulants) was allowed if the patient was on a stable dose. Most exposures were to aspirin. Antithrombotic medications did not increase the risk of ARIA with LEQEMBI. The incidence of ICH: 0.9% in patients taking LEQEMBI with a concomitant antithrombotic medication vs 0.6% with no antithrombotic and 2.5% in patients taking LEQEMBI with an anticoagulant alone or with antiplatelet medication such as aspirin vs none in patients receiving placebo.
Fatal cerebral hemorrhage has occurred in 1 patient taking an anti-amyloid monoclonal antibody in the setting of focal neurologic symptoms of ARIA and the use of a thrombolytic agent.
Additional caution should be exercised when considering the administration of antithrombotics or a thrombolytic agent (for example, tissue plasminogen activator) to a patient already being treated with LEQEMBI. Because ARIA-E can cause focal neurologic deficits that can mimic an ischemic stroke, treating clinicians should consider whether such symptoms could be due to ARIA-E before giving thrombolytic therapy in a patient being treated with LEQEMBI.
Caution should be exercised when considering the use of LEQEMBI in patients with factors that indicate an increased risk for ICH and, in particular, patients who need to be on anticoagulant therapy or patients with findings on MRI that are suggestive of CAA.
Radiographic Severity With LEQEMBI
Most ARIA-E radiographic events occurred within the first 7 doses, although ARIA can occur at any time and patients can have greater than 1 episode. Maximum radiographic severity of ARIA-E with LEQEMBI was mild in 4%, moderate in 7%, and severe in 1% of patients. Resolution on MRI occurred in 52% of ARIA-E patients by 12 weeks, 81% by 17 weeks, and 100% overall after detection. Maximum radiographic severity of ARIA-H microhemorrhage with LEQEMBI was mild in 9%, moderate in 2%, and severe in 3% of patients; superficial siderosis was mild in 4%, moderate in 1%, and severe in 0.4% of patients. With LEQEMBI, the rate of severe radiographic ARIA-E was highest in ApoE ε4 homozygotes (5%) vs heterozygotes (0.4%) or noncarriers (0%). With LEQEMBI, the rate of severe radiographic ARIA-H was highest in ApoE ε4 homozygotes (13.5%) vs heterozygotes (2.1%) or noncarriers (1.1%).
Monitoring and Dose Management Guidelines
Baseline brain MRI and periodic monitoring with MRI are recommended. Enhanced clinical vigilance for ARIA is recommended during the first 14 weeks of treatment. Depending on ARIA-E and ARIA-H clinical symptoms and radiographic severity, use clinical judgment when considering whether to continue dosing or to temporarily or permanently discontinue LEQEMBI. If a patient experiences ARIA symptoms, clinical evaluation should be performed, including MRI if indicated. If ARIA is observed on MRI, careful clinical evaluation should be performed prior to continuing treatment.
HYPERSENSITIVITY REACTIONS
Hypersensitivity reactions, including angioedema, bronchospasm, and anaphylaxis, have occurred with LEQEMBI. Promptly discontinue the infusion upon the first observation of any signs or symptoms consistent with a hypersensitivity reaction and initiate appropriate therapy.
INFUSION-RELATED REACTIONS (IRRs)
IRRs were observed—LEQEMBI: 26%; placebo: 7%—and most cases with LEQEMBI (75%) occurred with the first infusion. IRRs were mostly mild (69%) or moderate (28%). Symptoms included fever and flu-like symptoms (chills, generalized aches, feeling shaky, and joint pain), nausea, vomiting, hypotension, hypertension, and oxygen desaturation.
IRRs can occur during or after the completion of infusion. In the event of an IRR during the infusion, the infusion rate may be reduced or discontinued, and appropriate therapy initiated as clinically indicated. Consider prophylactic treatment prior to future infusions with antihistamines, acetaminophen, nonsteroidal anti-inflammatory drugs, or corticosteroids.
ADVERSE REACTIONS
The most common adverse reactions reported in greater than or equal to 5% with LEQEMBI infusion every 2 weeks and greater than or equal to 2% higher than placebo were IRRs (LEQEMBI: 26%; placebo: 7%), ARIA-H (LEQEMBI: 14%; placebo: 8%), ARIA-E (LEQEMBI: 13%; placebo: 2%), headache (LEQEMBI: 11%; placebo: 8%), superficial siderosis of central nervous system (LEQEMBI: 6%; placebo: 3%), rash (LEQEMBI: 6%; placebo: 4%), and nausea/vomiting (LEQEMBI: 6%; placebo: 4%)
Safety profile of LEQEMBI IQLIK for maintenance treatment was similar to LEQEMBI infusion. Patients who received LEQEMBI IQLIK experienced localized and systemic (less frequent) injection-related reactions (mild to moderate in severity)
LEQEMBI (lecanemab-irmb) is available:
Intravenous infusion: 100 mg/mL
Subcutaneous injection: 200 mg/mL