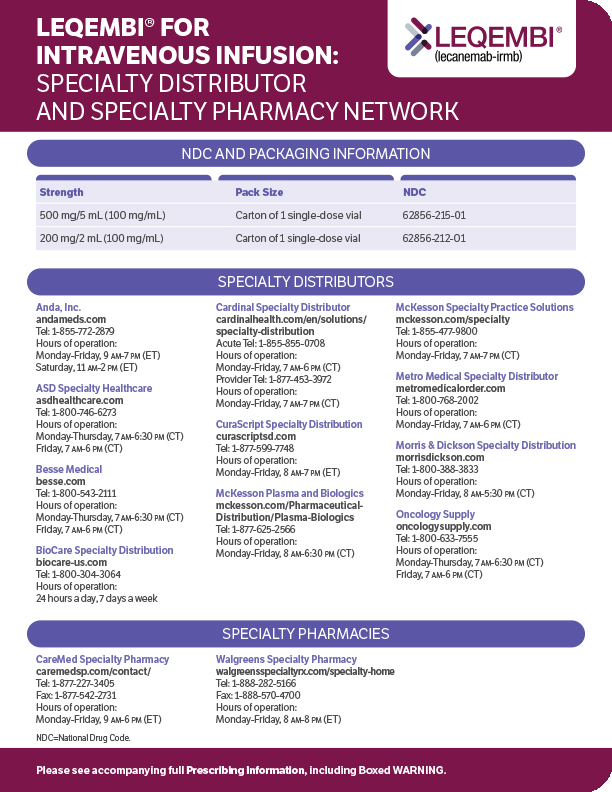
Only LEQEMBI empowers patients with
maintenance dosing options to help them
continue their treatment journey1,2
A baseline MRI is required to initiate treatment.1
*10 mg/kg once every 4 weeks. Patients may also continue with 10 mg/kg twice-monthly infusion dosing after 18 months.1
Only LEQEMBI offers titration-free dosing from the start—the recommended starting dosage is 10 mg/kg infusion over approximately 1 hour, twice monthly1,2
Throughout treatment, if a patient experiences symptoms suggestive of ARIA, clinical evaluation should be performed, including an MRI if indicated.1

Patients who continue on therapy beyond 18 months may be able to maintain treatment benefits for longer1
If transitioning from starting dosage to a maintenance dosage regimen, administer the first maintenance dose 2 weeks after the last starting dose.1
For patients with AD (MCI due to AD and mild AD dementia), LEQEMBI offers titration-free dosing from the start1
- Confirm the presence of Aβ pathology prior to initiating treatment
- After each infusion, ensure the patient/care partner has confirmed an appointment for their next infusion
- During maintenance dosage regimen, patients may switch the route of administration (intravenous LEQEMBI or subcutaneous LEQEMBI IQLIK). This transition should be initiated at 1 week following the last maintenance dose of either the intravenous or subcutaneous dosing regimen. Thereafter, follow the dosing schedule for the newly assigned maintenance dosage regimen
IV Administration1
Visually inspect the LEQEMBI diluted solution for particles or discoloration prior to administration. Do not use if it is discolored, or opaque, or foreign particles are seen
Prior to infusion, allow the LEQEMBI diluted solution to warm to room temperature
Infuse the entire volume of LEQEMBI diluted solution intravenously over approximately 1 hour through an IV line containing a terminal low-protein binding 0.2 micron in-line filter. Flush infusion line to ensure all LEQEMBI is administered
Monitor for any signs or symptoms of an infusion-related reaction. The infusion rate may be reduced, or the infusion may be discontinued, and appropriate therapy administered as clinically indicated. Consider pre-medication at subsequent dosing with antihistamines, non-steroidal anti-inflammatory drugs, or corticosteroids
If a starting dosage or maintenance dosage infusion is missed, administer the next dose as soon as possible
After each infusion, ensure the patient/care partner has confirmed an appointment for their next infusion
See dosing calculator below.


The first and only anti-amyloid treatment to offer an at-home
injection to help patients continue their treatment journey after
18 months1,2
Once-weekly at-home subcutaneous administration1

Prepare, inject in 15 seconds, and dispose1
Single-dose prefilled autoinjector: 360 mg/1.8 mL (200 mg/mL)1
For detailed information on how to prepare, administer, and safely dispose of LEQEMBI IQLIK,
review and advise the patient and/or care partner to read FDA-approved patient labeling
(Medication Guide and Instructions for Use)
How to Use LEQEMBI IQLIK
Is your patient starting LEQEMBI
at-home injections?
Share the video to help guide
them on how to use LEQEMBI
IQLIK safely and correctly.
LEQEMBI IQLIK administration1
Before injection, remove LEQEMBI IQLIK from the refrigerator and leave at room temperature for 20 minutes. Do not shake the autoinjector
Inspect LEQEMBI IQLIK for particles or discoloration prior to administration. The solution should be a clear to opalescent, colorless to pale yellow solution, and free of visible particles
Do not use LEQEMBI IQLIK if it is cloudy or there are visible particles. Do not use LEQEMBI IQLIK if it looks damaged or has been dropped
Sites for injection include the abdomen, upper thigh, and back of the upper arm
Do not inject into moles, scars, bruises, tattoos, or into areas where the skin is red, hard, tender, or injured
LEQEMBI IQLIK does not contain preservatives
During maintenance dosing, patients may switch from infusion (every 4 weeks) to subcutaneous LEQEMBI IQLIK (once weekly), or vice versa. Initiate this transition at 1 week after the last maintenance dose
Monitor for signs or symptoms of an injection reaction
Dosing calculator for IV administration
The information provided is not a substitute for clinical judgment.
This is intended for qualified healthcare providers only. All calculations should be confirmed before use.
Dosing for LEQEMBI is calculated using actual body weight. Use this dosing calculator to determine the exact volume of LEQEMBI needed for your patient.
Enter your patient’s actual weight in lb or kg and click ‘Calculate dose’ to determine the appropriate dose of LEQEMBI in mg/mL.
Based on the dosage calculated, follow the dilution instructions below in the full Prescribing Information.