This video will teach you how to use LEQEMBI® IQLIK™.
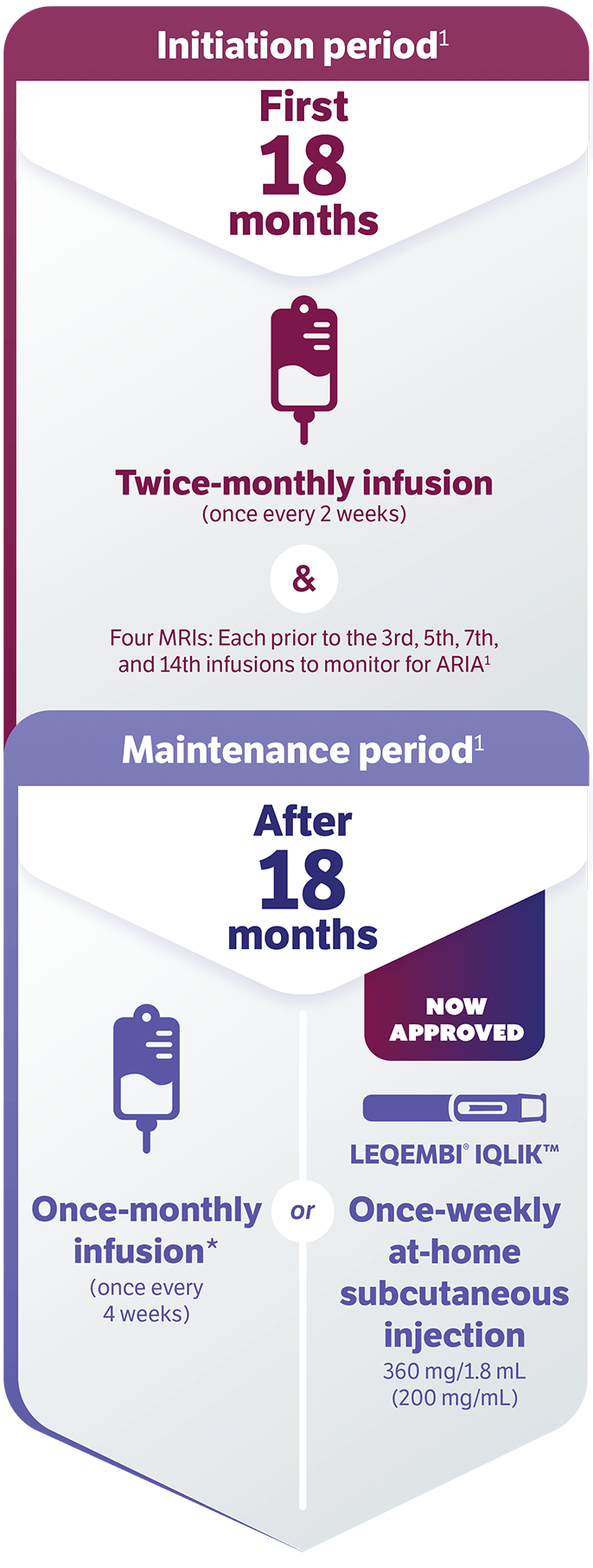
This once-weekly, at-home maintenance treatment is an available option to patients after 18 months of LEQEMBI infusions.
LEQEMBI is a prescription medicine used to treat people with Alzheimer's disease, which includes mild cognitive impairment (MCI) or mild dementia stage of disease.
Before you start using LEQEMBI IQLIK, read the Instructions for Use and the Medication Guide that you received with your autoinjector.
You should also read them each time you refill because there may be new information. This information does not take the place of talking to your health care provider about your medical condition or your treatment. If you have any questions about how to use the autoinjector, contact your health care provider.
Meet Sophia, she'll be demonstrating the injection process.
This is Sophia's husband, Rick. He's here to help.
Place your supplies on a clean, flat surface.
You'll need the LEQEMBI IQLIK autoinjector, cotton ball or gauze, an adhesive bandage, an alcohol wipe, and a sharps disposal container.
Your LEQEMBI IQLIK arrives in a refrigerated carton.
When you are ready to inject, take one out and let it sit at room temperature for 20 minutes.
Check the carton and autoinjector for damaged or broken seals.
Do not use if the expiration date on the carton has passed, or if the perforations on the carton are broken.
Check the viewing window. Do not use the autoinjector if it has been damaged or dropped, looks cloudy, discolored, or contains particles. The liquid should be clear and colorless to pale yellow. Air bubbles are normal.
Do not use if the expiration date has passed.
Once at room temperature, it can stay out for up to 14 days, but don't put it back in the fridge.
Use 1 injector weekly as directed.
Before you inject, wash your hands with soap and water, or use hand sanitizer.
Do not inject through clothing.
You can inject into the front of the thighs or stomach area.
If a care partner helps, they can also inject in the back of the upper arms.
Do not inject into moles, scars, bruises, tattoos, or red or injured skin. Do not inject into the 2-inch area around your belly button or through clothing.
Pick your site at least 1 inch away from your last injection.
Clean with an alcohol wipe and let it air dry.
Don't touch, fan, or blow after it's been cleaned.
Remove the clear cap by pulling it straight off. Don't twist or bend.
Throw the cap away in your trash or sharps container.
Place the magenta needle cover flat against the skin at a 90-degree angle.
Push down firmly to start the injection. Hold steady.
You may hear a click, which means the injection has started.
The purple plunger rod will move down in the viewing window during the injection.
If you have trouble hearing the click, watch the purple plunger rod as it moves down.
Hold for about 10 seconds.
You'll then hear a second click, which means the injection is complete. Keep holding for 5 more seconds.
Pull the autoinjector straight up. The magenta needle cover will automatically move into place to cover the needle.
You may notice a small drop of blood. This is normal. Press a cotton ball or gauze on the area and cover with a bandage if needed. Do not rub the injection site.
Throw away the used injector into a sharps container.
If you don't have an FDA-cleared sharps container, use a heavy-duty plastic container with a tight-fitting, puncture-resistant lid. Label it clearly.
When it's almost full, follow local guidelines to dispose of it properly.
That's it.
We've covered how to gather your supplies and prepare, inject, and dispose of LEQEMBI IQLIK.
You can watch this video again any time.
If you have questions, reach out to your health care provider.
Scan the QR code to download a wallet card that informs your health care provider you are taking LEQEMBI IQLIK.
INDICATION
LEQEMBI® is indicated for the treatment of Alzheimer's disease (AD). Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment (MCI) or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.
IMPORTANT SAFETY INFORMATION
WARNING: AMYLOID-RELATED IMAGING ABNORMALITIES (ARIA)
Monoclonal antibodies directed against aggregated forms of beta amyloid, including LEQEMBI, can cause ARIA, characterized as ARIA with edema (ARIA-E) and ARIA with hemosiderin deposition (ARIA-H). Incidence and timing of ARIA vary among treatments. ARIA usually occurs early in treatment and is usually asymptomatic, although serious and life-threatening events, including seizure and status epilepticus, can occur. ARIA can be fatal. Serious intracerebral hemorrhages (ICH) greater than 1 cm, some of which have been fatal, have been observed with this class of medications. Because ARIA-E can cause focal neurologic deficits that can mimic an ischemic stroke, consider whether such symptoms could be due to ARIA-E before giving thrombolytic therapy to a patient being treated with LEQEMBI.
Apolipoprotein E ε4 (ApoE ε4) Homozygotes: Patients who are ApoE ε4 homozygotes (approximately 15% of patients with AD) treated with this class of medications have a higher incidence of ARIA, including symptomatic, serious, and severe radiographic ARIA, compared to heterozygotes and noncarriers. Testing for ApoE ε4 status should be performed prior to initiation of treatment to inform the risk of developing ARIA. Prior to testing, prescribers should discuss with patients the risk of ARIA across genotypes and the implications of genetic testing results. Prescribers should inform patients that if genotype testing is not performed, they can still be treated with LEQEMBI; however, it cannot be determined if they are ApoE ε4 homozygotes and at higher risk for ARIA.
Consider the benefit of LEQEMBI for the treatment of AD and the potential risk of serious ARIA events when deciding to initiate treatment with LEQEMBI.
CONTRAINDICATION
Contraindicated in patients with serious hypersensitivity to lecanemab-irmb or to any of the excipients. Reactions have included angioedema and anaphylaxis.
WARNINGS AND PRECAUTIONS
AMYLOID-RELATED IMAGING ABNORMALITIES
Medications in this class, including LEQEMBI, can cause ARIA-E, which can be observed on MRI as brain edema or sulcal effusions, and ARIA-H, which includes microhemorrhage and superficial siderosis. ARIA can occur spontaneously in patients with AD, particularly in patients with MRI findings suggestive of cerebral amyloid angiopathy (CAA), such as pretreatment microhemorrhage or superficial siderosis. ARIA-H generally occurs with ARIA-E. Reported ARIA symptoms may include headache, confusion, visual changes, dizziness, nausea, and gait difficulty. Focal neurologic deficits may also occur. Symptoms usually resolve over time.
Incidence of ARIA
Symptomatic ARIA occurred in 3% and serious ARIA symptoms in 0.7% with LEQEMBI. Clinical ARIA symptoms resolved in 79% of patients during the period of observation. ARIA, including asymptomatic radiographic events, was observed: LEQEMBI, 21%; placebo, 9%. ARIA-E was observed: LEQEMBI, 13%; placebo, 2%. ARIA-H was observed: LEQEMBI, 17%; placebo, 9%. No increase in isolated ARIA-H was observed for LEQEMBI vs placebo.
Incidence of ICH
ICH greater than 1 cm in diameter was reported in 0.7% with LEQEMBI vs 0.1% with placebo. Fatal events of ICH in patients taking LEQEMBI have been observed.
Risk Factors of ARIA and ICH
ApoE ε4 Carrier Status
Of the patients taking LEQEMBI, 16% were ApoE ε4 homozygotes, 53% were heterozygotes, and 31% were noncarriers. With LEQEMBI, ARIA was higher in ApoE ε4 homozygotes (LEQEMBI: 45%; placebo: 22%) than in heterozygotes (LEQEMBI: 19%; placebo: 9%) and noncarriers (LEQEMBI: 13%; placebo: 4%). Symptomatic ARIA-E occurred in 9% of ApoE ε4 homozygotes vs 2% of heterozygotes and 1% of noncarriers. Serious ARIA events occurred in 3% of ApoE ε4 homozygotes and in approximately 1% of heterozygotes and noncarriers. The recommendations on management of ARIA do not differ between ApoE ε4 carriers and noncarriers.
Radiographic Findings of CAA
Neuroimaging findings that may indicate CAA include evidence of prior ICH, cerebral microhemorrhage, and cortical superficial siderosis. CAA has an increased risk for ICH. The presence of an ApoE ε4 allele is also associated with CAA.
The baseline presence of at least 2 microhemorrhages or the presence of at least 1 area of superficial siderosis on MRI, which may be suggestive of CAA, have been identified as risk factors for ARIA. Patients were excluded from Clarity AD for the presence of greater than 4 microhemorrhages and additional findings suggestive of CAA (prior cerebral hemorrhage greater than 1 cm in greatest diameter, superficial siderosis, vasogenic edema) or other lesions (aneurysm, vascular malformation) that could potentially increase the risk of ICH.
Concomitant Antithrombotic or Thrombolytic Medication
In Clarity AD, baseline use of antithrombotic medication (aspirin, other antiplatelets, or anticoagulants) was allowed if the patient was on a stable dose. Most exposures were to aspirin. Antithrombotic medications did not increase the risk of ARIA with LEQEMBI. The incidence of ICH: 0.9% in patients taking LEQEMBI with a concomitant antithrombotic medication vs 0.6% with no antithrombotic and 2.5% in patients taking LEQEMBI with an anticoagulant alone or with antiplatelet medication such as aspirin vs none in patients receiving placebo.
Fatal cerebral hemorrhage has occurred in 1 patient taking an anti-amyloid monoclonal antibody in the setting of focal neurologic symptoms of ARIA and the use of a thrombolytic agent.
Additional caution should be exercised when considering the administration of antithrombotics or a thrombolytic agent (for example, tissue plasminogen activator) to a patient already being treated with LEQEMBI. Because ARIA-E can cause focal neurologic deficits that can mimic an ischemic stroke, treating clinicians should consider whether such symptoms could be due to ARIA-E before giving thrombolytic therapy in a patient being treated with LEQEMBI.
Caution should be exercised when considering the use of LEQEMBI in patients with factors that indicate an increased risk for ICH and, in particular, patients who need to be on anticoagulant therapy or patients with findings on MRI that are suggestive of CAA.
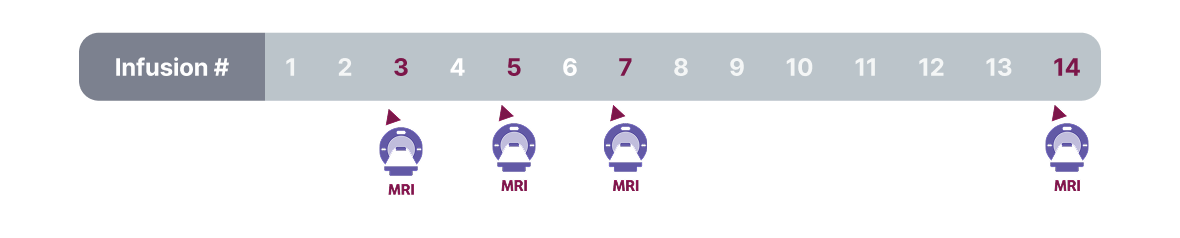
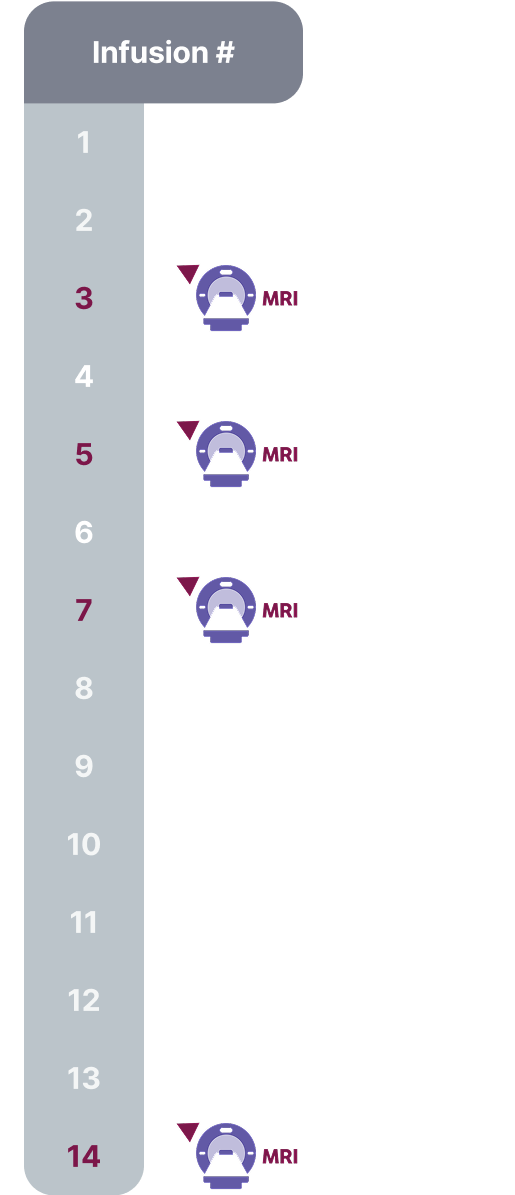
Radiographic Severity With LEQEMBI
Most ARIA-E radiographic events occurred within the first 7 doses, although ARIA can occur at any time and patients can have greater than 1 episode. Maximum radiographic severity of ARIA-E with LEQEMBI was mild in 4%, moderate in 7%, and severe in 1% of patients. Resolution on MRI occurred in 52% of ARIA-E patients by 12 weeks, 81% by 17 weeks, and 100% overall after detection. Maximum radiographic severity of ARIA-H microhemorrhage with LEQEMBI was mild in 9%, moderate in 2%, and severe in 3% of patients; superficial siderosis was mild in 4%, moderate in 1%, and severe in 0.4% of patients. With LEQEMBI, the rate of severe radiographic ARIA-E was highest in ApoE ε4 homozygotes (5%) vs heterozygotes (0.4%) or noncarriers (0%). With LEQEMBI, the rate of severe radiographic ARIA-H was highest in ApoE ε4 homozygotes (13.5%) vs heterozygotes (2.1%) or noncarriers (1.1%).
Monitoring and Dose Management Guidelines
Baseline brain MRI and periodic monitoring with MRI are recommended. Enhanced clinical vigilance for ARIA is recommended during the first 14 weeks of treatment. Depending on ARIA-E and ARIA-H clinical symptoms and radiographic severity, use clinical judgment when considering whether to continue dosing or to temporarily or permanently discontinue LEQEMBI. If a patient experiences ARIA symptoms, clinical evaluation should be performed, including MRI if indicated. If ARIA is observed on MRI, careful clinical evaluation should be performed prior to continuing treatment.
HYPERSENSITIVITY REACTIONS
Hypersensitivity reactions, including angioedema, bronchospasm, and anaphylaxis, have occurred with LEQEMBI. Promptly discontinue the infusion upon the first observation of any signs or symptoms consistent with a hypersensitivity reaction and initiate appropriate therapy.
INFUSION-RELATED REACTIONS (IRRs)
IRRs were observed—LEQEMBI: 26%; placebo: 7%—and most cases with LEQEMBI (75%) occurred with the first infusion. IRRs were mostly mild (69%) or moderate (28%). Symptoms included fever and flu-like symptoms (chills, generalized aches, feeling shaky, and joint pain), nausea, vomiting, hypotension, hypertension, and oxygen desaturation.
IRRs can occur during or after the completion of infusion. In the event of an IRR during the infusion, the infusion rate may be reduced or discontinued, and appropriate therapy initiated as clinically indicated. Consider prophylactic treatment prior to future infusions with antihistamines, acetaminophen, nonsteroidal anti-inflammatory drugs, or corticosteroids.
ADVERSE REACTIONS
The most common adverse reactions reported in greater than or equal to 5% with LEQEMBI infusion every 2 weeks and greater than or equal to 2% higher than placebo were IRRs (LEQEMBI: 26%; placebo: 7%), ARIA-H (LEQEMBI: 14%; placebo: 8%), ARIA-E (LEQEMBI: 13%; placebo: 2%), headache (LEQEMBI: 11%; placebo: 8%), superficial siderosis of central nervous system (LEQEMBI: 6%; placebo: 3%), rash (LEQEMBI: 6%; placebo: 4%), and nausea/vomiting (LEQEMBI: 6%; placebo: 4%)
Safety profile of LEQEMBI IQLIK for maintenance treatment was similar to LEQEMBI infusion. Patients who received LEQEMBI IQLIK experienced localized and systemic (less frequent) injection-related reactions (mild to moderate in severity)
LEQEMBI (lecanemab-irmb) is available:
Intravenous infusion: 100 mg/mL
Subcutaneous injection: 200 mg/mL